Roweepra

Roweepra® is OWP Pharmaceuticals’ branded generic alternative (AB rated) to Keppra® (levetiracetam) immediate-release tablets.*
Written DAW-1
Cost Affordability
Helping Others
Why Choose ROWEEPRA®?
ROWEEPRA® may be prescribed “DAW-1” (Dispense As Written – or appropriate language required by your state).
- This ensures consistency in receiving the prescribed medication from the same drug manufacturer at every refill.
- As long as the prescribed dosage remains consistent, ROWEEPRA® tablets are the same shape, size, and color at every refill.
ROWEEPRA® is available at a significantly lower cost than Keppra®.†
A portion of the profits from each ROWEEPRA® prescription help support the humanitarian work of the ROW Foundation, an organization dedicated to help bring training, diagnostics, and treatment for epilepsy and associated psychiatric disorders to under-resourced communities around the world.
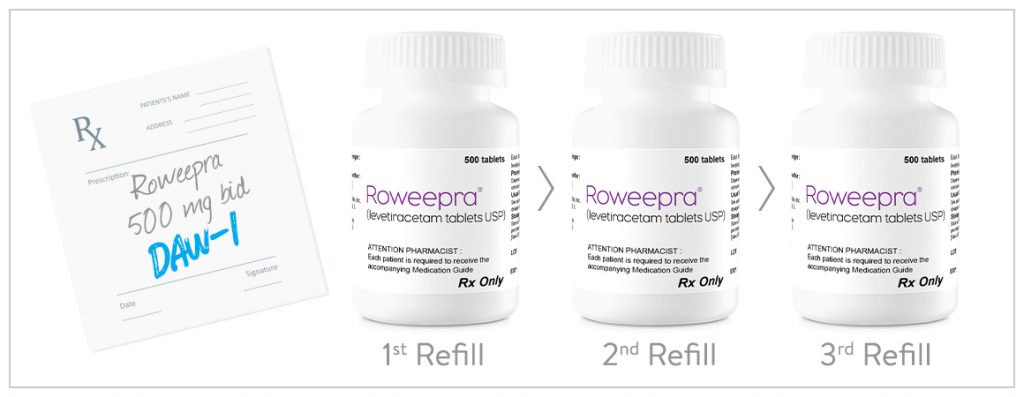
CONSISTENT MANUFACTURER (WITH DAW-1)

VARIED MANUFACTURERS (WITHOUT DAW-1)
Talk to your prescribing physician to see if ROWEEPRA® may be right for you.
If you are a patient with a prescription for ROWEEPRA®, it can be purchased through your local pharmacy. If you need help finding a pharmacy in your area that carries ROWEEPRA® please visit: https://owppharma.com/pharmacy-locator or call 800-273-6729 for assistance locating one.
*Keppra® is a registered trademark of UCB Biopharma
†https://www.goodrx.com/levetiracetam. Accessed 1-25-2019
For Roweepra questions please contact us at: medinfo@owppharma.com
To report safety-related concerns please contact us at: safety@owppharma.com


